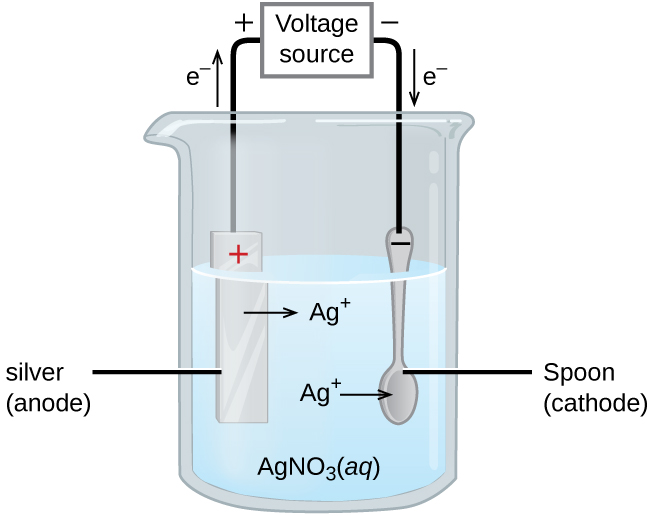
HULUSUL PURCULUI 13.A Copper-silver cell is up. The copper ion concentration in it is 0.10M.The concentration of silver is not known. The cell potential measured is 0.422V.Determine the concentration of silver ion

7. of A copper silver cell is setup. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measures 0.422V. Determine the
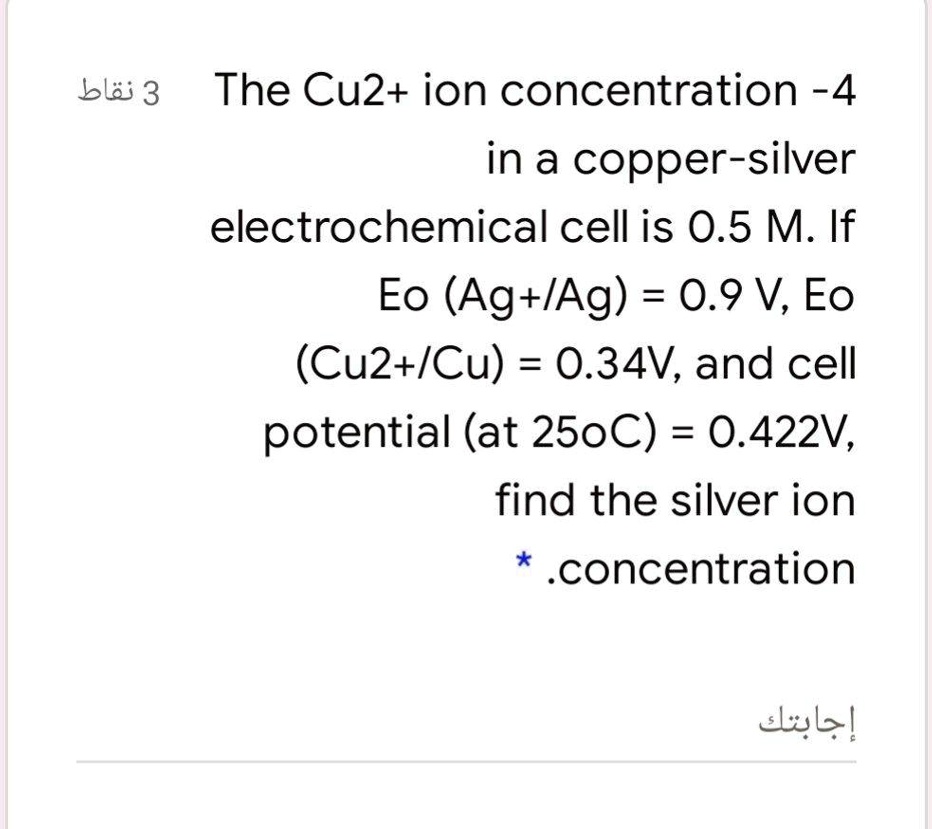
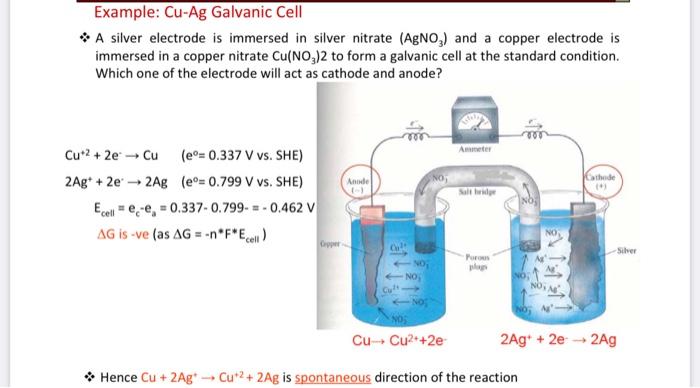
SOLVED: The Cu2+ ion concentration in a copper-silver electrochemical cell is 0.5 M. If Eo (Ag+/Ag) = 0.9 V, Eo (Cu2+/Cu) = 0.34 V, and cell potential (at 25°C) = 0.422 V,

The electrochemical cell for EMF measurement of chains of type (4.1)... | Download Scientific Diagram

A copper - silver cell is set up. The copper ion concentrations is 0.10 M. The concentration of... - YouTube
Dual hydrogen production from electrocatalytic water reduction coupled with formaldehyde oxidation via a copper-silver electrocatalyst | Nature Communications
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. - Sarthaks eConnect | Largest Online Education Community

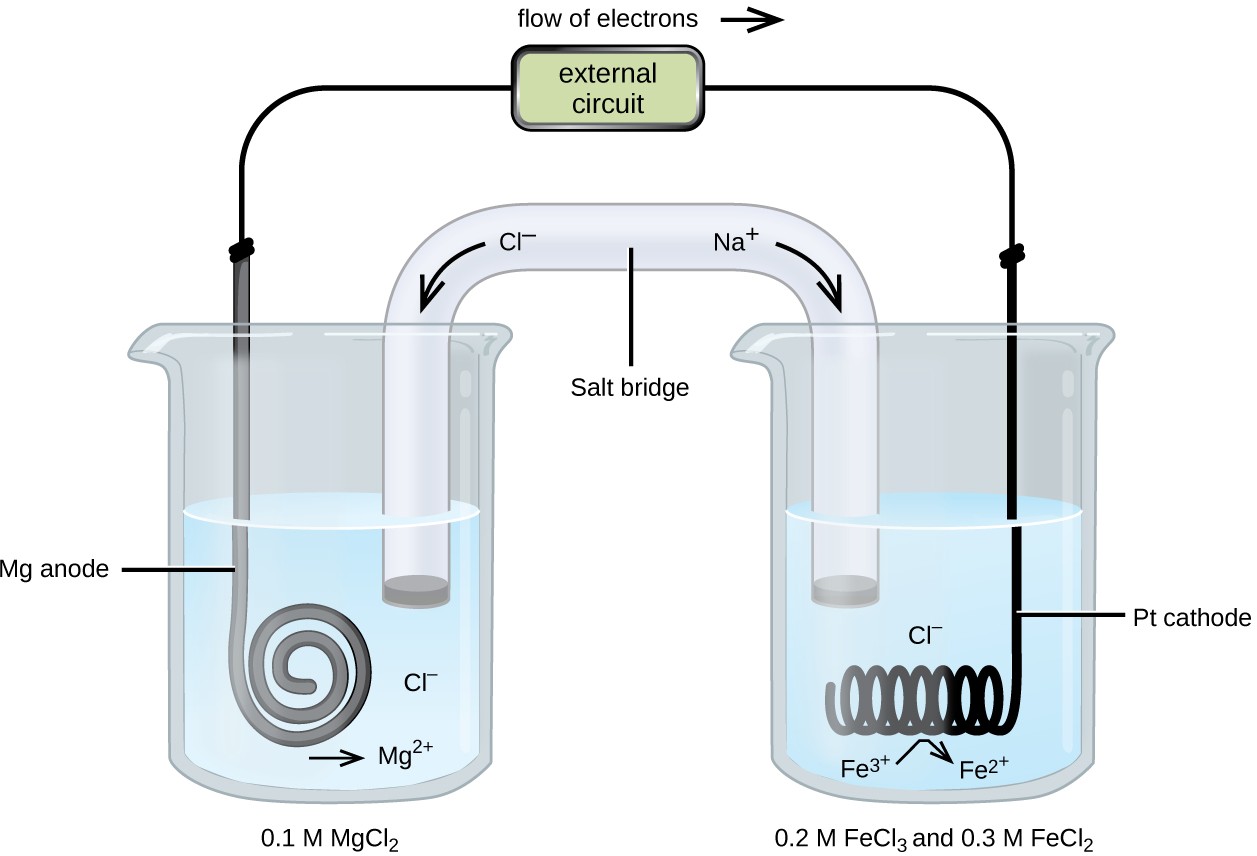
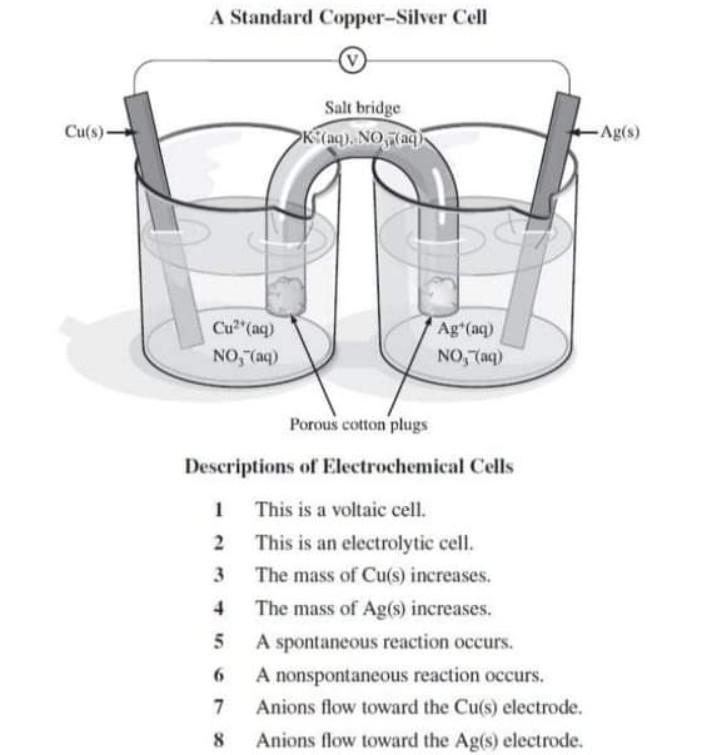
III. Cell Potentials Under Standard Conditions B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that includes all the components (

13.A copper-silver cell is up. The copperion concentration is 0.10M. The concentration of Silver ions is not known. The cell potential was found to be 0.422V.Determine the concentration Silver ion in

Following cell is set up between copper and silver electrodes Cu//Cu^(2+)(aq)||Ag^(+)//Ag. If tw... - YouTube
A copper_ silver cell is set up. The copper ion concentration is 0.10 M. The concentration of silver ion is not know.the cell potential when measured was 0.422V determine the concentration of
A copper- silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measured 0.422 V. Determine the

SOLVED: The Cu2+ ion concentration in a copper-silver electrochemical cell is 0.5 M. If Eo (Ag+/Ag) = 0.9 V, Eo (Cu2+/Cu) = 0.34 V, and cell potential (at 25°C) = 0.422 V,
BIM objects - Free download! Copper-Silver Ionization System - CSI - 2 Flow Cell 2 Controller Rack Mount - 2F2C | BIMobject

13.A copper-silver cell is up. The copperion concentration is 0.10M. The concentration of Silver ions is not known. The cell potential was found to be 0.422V.Determine the concentration Silver ion in





![Assamese] A copper silver cell is set up. The copper ion concentrati Assamese] A copper silver cell is set up. The copper ion concentrati](https://static.doubtnut.com/ss/web-overlay-thumb/8484396.webp)